Introduction
Global Medical Device Testing Market, a recently published in-depth analysis from Data Bridge Market Research, ensures that you will always be more knowledgeable than your rivals. With its thorough industry insights and research, this report offers a more comprehensive view of the market, making it easier for businesses to survive and succeed there. This medical device testing report has been created, which benefits the businesses in the form of great growth and strong market sustainability. This is as a result of the incorporation of accurate information and market research-derived insights.
What is medical device testing?
The process of proving a medical device’s dependability and safety in usage is known as testing. Extensive design validation testing is used in new product development. This covers performance testing, chemical analysis and toxicity testing, and occasionally human aspects or even clinical testing. Continuous quality assurance testing often has less resources. Dimensional checks, certain functional tests, and package verification are typically included. There are many different kinds of medical testing services on the market, including inspection services and certification services.
Market Dynamics of Medical Device Testing
- In the healthcare sector, verification and validation techniques are widely and extensively employed. In general, validation determines if a product has been used for its intended purpose and, as a result, whether usability standards have been satisfied. Verification, on the other hand, determines whether a product has been developed to the given requirements. Design, process, and software verification and validation are the most typical methods of verification and validation for medical devices.
- Additionally, the size and complexity of medical gadgets are increasing, and they occasionally make use of sophisticated, manufactured plastics. This increases the significance of validation and verification (V&V). Better repeatability, fewer errors, less need for rework and redesign, quicker time to market, increased competitiveness, and reduced manufacturing costs are the end results.
- Tests on human body samples like blood or tissue are known as in vitro diagnostics (IVD). In-vitro diagnostics can be used to monitor a person’s overall health, diagnose diseases or other disorders, and assist treat, prevent, or cure illnesses. In-vitro tests are used to diagnose a number of diseases, including hepatitis, malaria, and HIV infections. Globally, the prevalence of these diseases is spreading quickly, which is driving up demand for in-vitro diagnostics and other medical equipment.
- The speed of innovation advancement in the healthcare industry has greatly accelerated in recent years. Medical gadget technological advancements allow simple, painless therapy during illness management. Additionally, advancements in medical device technology help with accurate and quick illness diagnosis results, as well as the affordability of technology-based therapeutic instruments for disease treatment.
- Furthermore, the medical research facilities are supported by several governmental agencies and healthcare institutions. This funding is primarily intended to promote global health care advances. The expansion of medical device testing in the anticipated term is therefore supported by expanding innovation and technical progress.
Scope of medical device testing market
Significant industry trends, market size and share projections, and other subjects are all examined and discussed in this report. This study offers a benefit that enables one to not only compete with but also outperform one’s competitors.
It divides the size of the worldwide market for medical device testing into several categories based on the kinds of manufacturers, uses, and geographical locations. During the projected period, the outlook for the medical device testing market and the industry as a whole seems to be relatively favourable.
For more detail about Scope of medical device testing market visit
https://www.databridgemarketresearch.com/reports/global-medical-device-testing-market
Opportunity in medical device testing market
Worldwide healthcare spending has grown as people’s disposable income rises in various nations. Additionally, the government and healthcare institutions are taking the lead by increasing healthcare spending in order to meet the population’s needs. The increase in healthcare spending has recently assisted healthcare facilities in providing better medical device testing services.
Additionally, the structural integrity and future potential for the medical device testing market during the forecast period of 2022-2029 will be provided by leading market participants’ strategic activities.
Market size
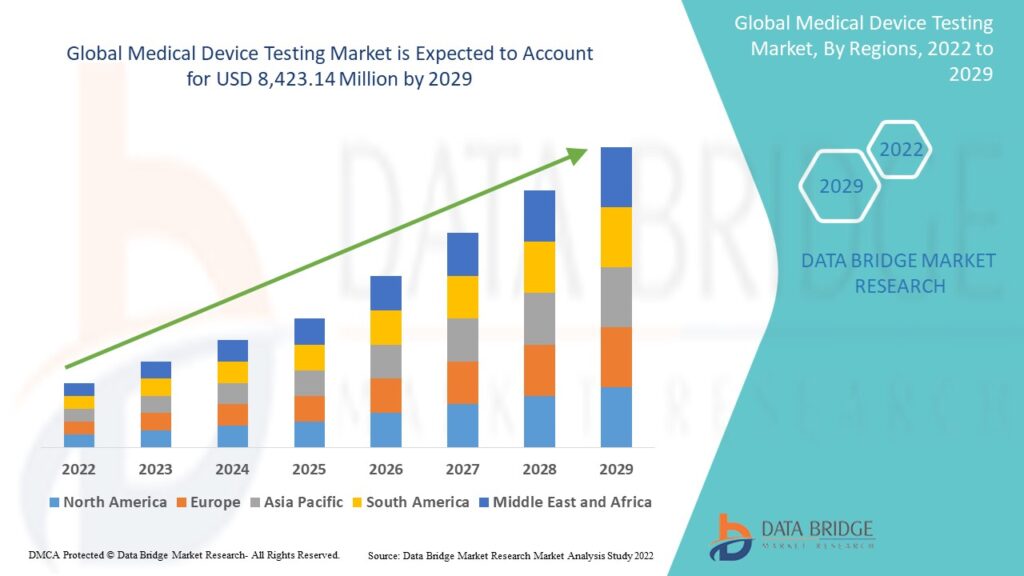
- The global market for testing medical devices is anticipated to expand from 2022 to 2029. According to Data Bridge Market Research’s analysis, the market is expanding at a CAGR of 10.8% from 2022 to 2029 and is expected to grow from USD 3,832.27 million in 2021 to USD 8,423.14 million by that time.
- The process of proving a medical device’s dependability and safety in usage is known as testing. Extensive design validation testing is used in new product development. This covers performance testing, chemical analysis and toxicity testing, and occasionally human aspects or even clinical testing. Continuous quality assurance testing often has less resources. Dimensional checks, certain functional tests, and package verification are typically included. There are many different kinds of medical testing services on the market, including inspection and certification services.
About Us
Data Bridge Market Research offers quantified B2B high-quality research on more than 20,000 emerging markets, assisting our clients in identifying the constellation of business opportunities. We offer comprehensive solutions to our client companies in order to help them achieve their important business goals. We are a competitive intelligence market research and consulting agency.